YUTIQ® (fluocinolone acetonide intravitreal implant) 0.18 mg is indicated for the treatment of chronic non-infectious uveitis affecting the posterior segment of the eye.
| VA | Imaging | |
|---|---|---|
| Before YUTIQ Treatment | 20/70 | 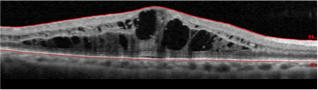 |
| 1-month follow-up after YUTIQ | 20/40 |  |
| 2-month follow-up after YUTIQ | 20/25 |  |
| 3-month follow-up after YUTIQ | 20/25 |  |
| 5-month follow-up after YUTIQ | 20/25 |  |
Hypersensitivity: YUTIQ is contraindicated in patients with known hypersensitivity to any components of this product.
Intravitreal Injection-related Effects: Intravitreal injections, including those with YUTIQ, have been associated with endophthalmitis, eye inflammation, increased or decreased intraocular pressure, and choroidal or retinal detachments. Hypotony has been observed within 24 hours of injection and has resolved within 2 weeks. Patients should be monitored following the intravitreal injection.
Steroid-related Effects: Use of corticosteroids including YUTIQ may produce posterior subcapsular cataracts, increased intraocular pressure and glaucoma. Use of corticosteroids may enhance the establishment of secondary ocular infections due to bacteria, fungi, or viruses. Corticosteroids are not recommended to be used in patients with a history of ocular herpes simplex because of the potential for reactivation of the viral infection.
Risk of Implant Migration: Patients in whom the posterior capsule of the lens is absent or has a tear are at risk of implant migration into the anterior chamber.
In controlled studies, the most common adverse reactions reported were cataract development and increases in intraocular pressure.
Please see full Prescribing Information.
©2022 EyePoint Pharmaceuticals, Inc. All rights reserved.
480 Pleasant Street, Suite B300, Watertown, MA 02472
YUTIQ, the YUTIQ logo, and the EyePoint logo are registered trademarks of EyePoint Pharmaceuticals, Inc.
04/2022 US-YUT-2200002